Pioneers of medical grade gelatins and collagens
Rousselot Biomedical is committed to deliver biomaterials that improve patients’ lives. Our team of experts combines cutting-edge technology with 130 years of gelatin and collagen experience to create customized gelatin solutions tailored to specific applications. We work closely with our customers to understand their unique requirements, providing them with the products and support they need to help them overcome their challenges. Rousselot is the partner of choice to leverage the power of gelatin-based biomaterials to drive advancements in biomedical applications, from pharmaceuticals to medical devices to cell therapy.
Products
Rousselot designs biomaterials to help you achieve your goals. We produce gelatins and modified gelatins for in-body applications.
Use our product finder to find your X-Pure® or Quali-Pure® gelatin solution today.


X-Pure® is our range of premium-grade modified and non-modified gelatins for research and in-body applications, with ultra-low endotoxins (≤10 EU/g) for maximum quality and safety.


Quali-Pure® is our range of endotoxin-controlled gelling and non-gelling gelatins with guaranteed biocompatibility, biodegradability, and batch-to-batch consistency.
Biomedical applications of gelatin
Researchers and pharmaceutical companies are using X-Pure® to advance a wide range of biomedical applications. Discover how our ultra-purified, GMP-grade gelatins and GelMAs can support your regenerative medicine, drug delivery, embolization and hemostatic projects.
Find your solutions with us
Order a Sample

Are you a researcher looking for
high quality gelatins?
Try our X-Pure gelatins and modified gelatins
(GelMA, GelDAT…)
Or Ask for Customized Solutions

Do you need tailored options for your
application with expert support?
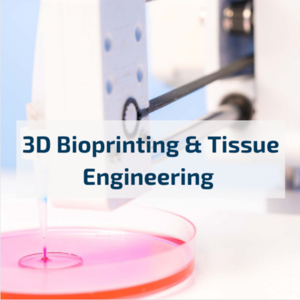
Recreate in vivo conditions and leverage the power of 3D cell culture with X-Pure®
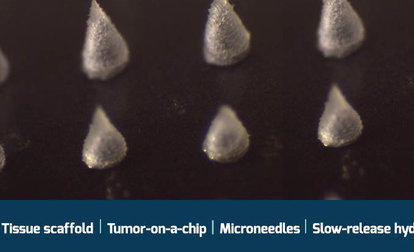
The molecular weight and degree of modification of X-Pure® gelatins and GelMAs can be customized to mimic the biochemical and mechanical properties of the native extracellular microenvironment, making them suitable for a wide range of biomedical applications, such as scaffolds, organoids, bioprinting and organs-on-a-chip.
Why choose Rousselot?
We do more than just produce and supply world-class biomedical gelatins. Gelatin is a high-performance biomaterial that reveals its full power when tailored to specific needs. The expertise of our worldwide teams enables us to provide our customers with personalized, effective solutions and to support them in their development.
Quality & Purity
Our gelatins are produced to GMP standard*, and our patented endotoxin removal process can help prevent an immune response. Find out more.
Services
We enable customers to engineer, accelerate and scale superior products, helping transform ideas into biomedical solutions worldwide. Find out more.
Technical Support
We rapidly respond to customer queries and our technical support experts ensure customer products deliver the impact intended. Find out more.
Custom Formulation Support
Our products can be customized to the ideal specification required for each application. Our products are produced in a standardized, purified, traceable and consistent manner, at the scale required.
Find out more.
Regulatory Support
We follow the latest guidance from regulatory bodies around the world, ensuring compliance at every stage. All our products include documented traceability according to ISO 22442 requirements, extended document retention and validated viral inactivation.Find out more.
Supply Chain Security
Rousselot's global presence and long history working with gelatin and collagens, coupled with our unique processing, ensure a secure supply chain to meet your needs at scale. Find out more.
This makes news
Meet us at events
DISCLAIMER:
Rousselot makes no representation or warranty, whether expressed or implied, as to the accuracy, reliability, or completeness of the information, nor does it assume any legal liability, whether direct or indirect, for any information. Use of this information shall be at your discretion and risk. Nothing herein relieves you from carrying out your own suitability determinations and tests and from your obligation to comply with all applicable laws and regulations and to observe all third-party rights. This product is not intended to diagnose, treat, cure, or prevent any disease.
* IPEC. Excipient Good Manufacturing Practices Guide, 2022