Implantable Medical Textiles and Membranes
Implantable medical textiles made from bioabsorbable materials can provide scaffolds and support to tissue following trauma or surgical procedures. Implantable medical textiles, woven or non-woven, prepared from gelatin are designed to mimic the properties of the body’s own extracellular matrix and are, therefore, biocompatible, providing favourable cell growth conditions. Moreover, they can be tailored to be bioabsorbable, thus avoiding additional procedures to remove them. However, endotoxins in these textiles and membranes can cause unwanted immune responses.
Gelatin is formed by the partial hydrolysis of collagen, the most abundant macromolecule of the extracellular matrix, and has a long history of use as a trusted excipient in pharmaceutical applications due to its versatility and inherent biocompatibility. However, the literature is full of examples of regenerative medicine applications for which gelatin was chosen as an ideal base material, but where translation into the clinic is complicated by undesired endotoxin contamination/side effects. Endotoxins or lipopolysaccharides are highly immunogenic components of the outer membrane of gram-negative bacteria that the human immune system is geared towards detecting and reacting to, even in minute quantities. Correspondingly, the FDA has imposed restrictions on endotoxin content in high-risk medical device applications at levels where traditionally manufactured gelatin with retained gelling characteristics are typically more than 10 times higher than what the FDA recommend.
However, one method can be used to remove endotoxins from gelatin that works for both type A and type B gelatin and retains the full spectrum of physical properties. This innovative approach is patented and suitable for Good Manufacturing Practice production and implementation.
The difference is remarkable. An experiment was recently conducted that compared a standard endotoxin reduction approach, using autoclaving process at 121°C and one bar for 30 minutes, with an in-house developed method. The gelatin was a typical type A, acquired from Sigma-Aldrich (G1890) with 20,000 endotoxin units per gram (EU/g). The results showed a 98% reduction in endotoxin levels using the autoclaving process, ie, from 20,000 EU/g down to 410 EU/g, measured using the EndoZyme recombinant Factor C method. However, this reduction in endotoxin levels came at the loss of gel strength (-60% − 297 to 120 bloom) and average molecular weight (-50% − 150kDa to 75kDa). Using this approach, the purified gelatin retained an average gel strength of 310 bloom and an average molecular weight of 147kDa, despite a greater than
99.9% reduction in endotoxin levels.
To illustrate the potential impact from endotoxins in gelatin on cell behaviour, the mitochondrial activity and cell growth characteristics of an endotoxin-sensitive cell line was evaluated. In the experiment, a 1% gelatin
solution of a non-purified Sigma-Aldrich G1890 gelatin was compared to a 1% purified gelatin solution before and after endotoxin reduction.
When using the purified gelatins, the mitochondrial activity and growth of the cells were much higher compared to the non-purified gelatin.
Five regenerative medicine application areas where gelatin offers key benefits over other biomaterials can be identified, in which the presence of endotoxins complicate the development of successful applications:
- Implantable medical textiles and membranes
- Wound healing
- (Stem) cell and organoid culturing
- Targeted drug delivery
- 3D bioprinting and tissue engineering
Endotoxin is a common cause of complications in surgery, resulting in 40,000 out of 500,000 annual arthroplasty revisions due to the aseptic loosening in the US alone (1)
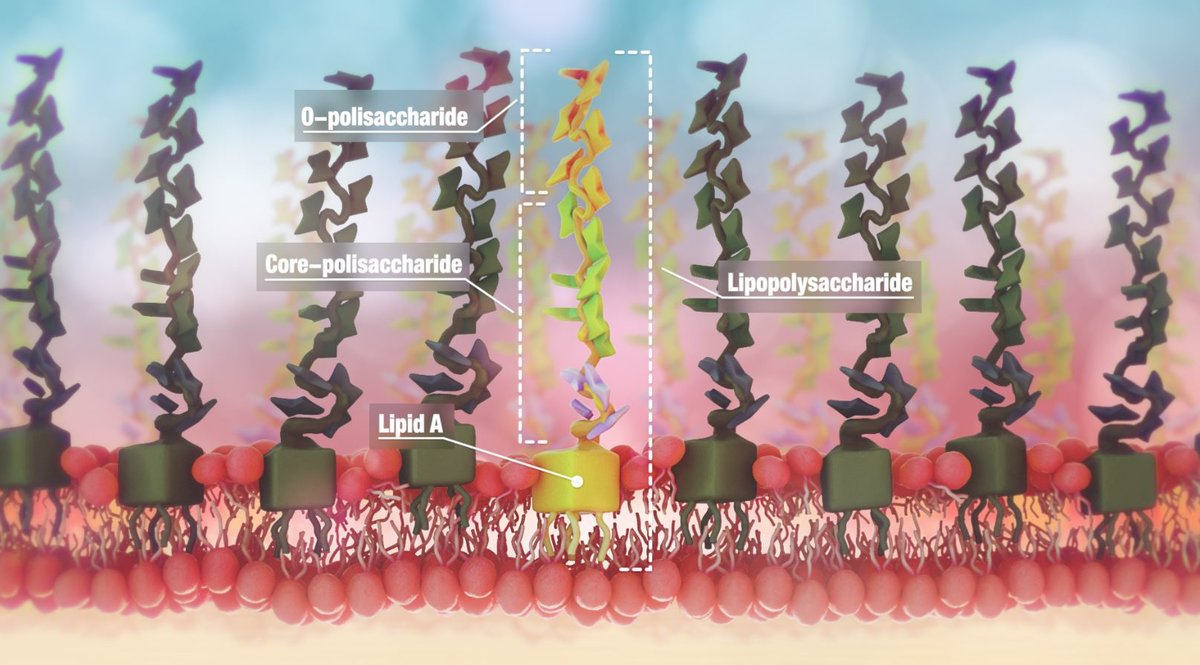
Endotoxin structure
Figure 1: Endotoxin structure
While low endotoxin levels in implanted medical textiles will not entirely eliminate endotoxin contamination in surgery, the use of low endotoxin gelatin as the base ingredient in implantable medical textiles will aid in
minimising the overall endotoxin load during and, following surgery, helping reduce the risk of sustained inflammation and delayed formation of new healthy tissue.
Wound healing
Wound care poses one of the greatest challenges for healthcare today, with non-healing and complicated wounds costing more than the total cost of care for asthma, dementia, or obesity (2). Gelatin with or without additional materials has been consistently demonstrated as a suitable material for use in skin grafts and scaffolds to enhance epithelialisation in wounds (3). Prolonged inflammation is often a complication preventing timely wound healing. As such, minimising additional proinflammatory stimuli from endotoxins within grafts and scaffolds could materially reduce the risk of sustained inflammation.
Stem cell manufacturing
Stem cell culturing has proven to be difficult, with challenges in maintaining viability and achieving efficient differentiation. Gelatin has been shown to be a medium in which stem cells can successfully grow and differentiate. However, stem cells demonstrate host defense responses even in the presence of trace amounts of endotoxins (4). For example, endotoxins have been found to inhibit osteoblast differentiation at doses
greater than 100ng/ml (5). With low endotoxin gelatin now available, leveraging the benefits of gelatin in a large range of stem cell applications without concern for the negative impact from endotoxins is possible.
Drug delivery
Gelatin has demonstrated great versatility with usage across a wide range of drug delivery systems, including encapsulation in micro- and nanospheres, incorporation into gel films, ocular inserts, and eye drops, providing great flexibility in drug release profiles. Moreover, drugs with otherwise poor bioavailability have been successfully formulated with gelatin (3). However, in certain drug delivery applications, endotoxins can cause elevated injection site reactions. In extreme cases, even death is possible from endotoxin-induced immune responses. With low endotoxin gelatin now available, minimising the risk of endotoxin-induced immune responses is possible.
Bioprinting
Nanomedicine and 3D printing are receiving significant attention and investment, based on the prospects of creating customised organ-on-chip designs to allow for reduced reliance on animal testing, as well as 3D printed scaffolds for replacing human tissue. For example, combined with methacrylamide, gelatin can be used to create scaffolds of virtually any shape and with a large range of mechanical properties. Additionally, such scaffolds can be engineered for a controlled release of a variety of biomolecules (4). In such applications, minimising the risk of the new bioengineered tissue getting rejected via an immune system response is mportant. With low endotoxin gelatin now available, it is possible to produce such 3D printed biomaterials with a low risk of immune reactions to endotoxins within the scaffold.

Rousselot X-Pure
In all of these applications, gelatin offers attractive physical properties such as adjustable gel strength, high biocompatibility, and bioabsorption. With low endotoxin gelatin now available, it has become possible to choose the right base material for bioengineering applications where consistently low immunogenicity is desirable.
References
1. Goveia VR et al, Endotoxins in surgical instruments of hip arthroplasty, Rev Esc Enferm 50(3): pp405-10, 2016
2. Visit: pressureinjuryprevention.com/uk-wound-care-cost/
3. Nikkhah M et al, Gelatin-based biomaterials for tissue engineering and stem cell bioengineering, Biomaterials from Nature for Advanced Devices and Therapies 1st edition: pp37-62, 2016
4. Numura Y et al, A biological study establishing the endotoxin limit for in vitro proliferation of human mesenchymal stem cells, Regenerative Therapy 7: pp45-51, 2017
5. Kadono H et al, Inhibition of osteoblastic cell differentiation by lipopolyaccaride extract from porphymona gingivalis, Infect Immun 67(6): pp2,841-6, 1999
About the author

Barbara Vanhoecke is the Innovation Manager Biomedical at Rousselot, working on the Rousselot X-Pure range in particular. Barbara has published two patent applications and is author/co-author of more than 40 scientific papers. She holds a PhD in Medical Sciences and a Masters in Biochemistry.
![[Translate to Portuguese:] X-Pure gelatin on biomedical applications [Translate to Portuguese:] X-Pure gelatin on biomedical applications](https://d1ip4j1950xau.cloudfront.net/_processed_/5/a/csm_X%20Pure%20view%20from%20the%20bench_4eeda7e77c.jpg)