The fundamental aim of biomedical research is to find new or alternative solutions to treat diseases and improve patients’ lives. But translating early discoveries into effective treatments is time-consuming, costly and often unsuccessful. New treatments can fail for many reasons, and those that are successful typically go through many rounds of iterations before they reach the market. Overall, the average time required for end-to-end translation, from an idea in the laboratory to a drug or final product for patients, is currently over 20 years, and the success rate is below 1%i. It’s therefore no surprise that the phase between research and successful commercialization is known as “the valley of death.”
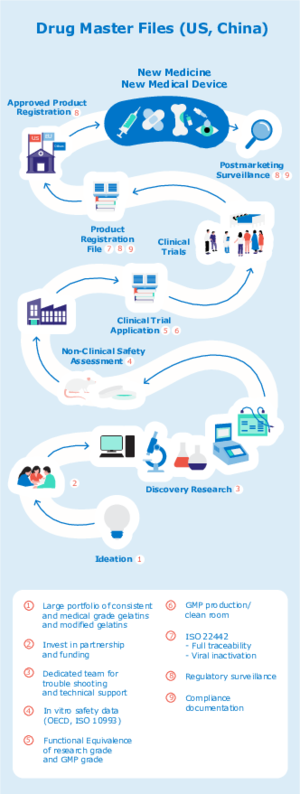
Bridging the valley of death
Despite the treacherous route to market, there are several ways to improve the outcomes of research-to-clinic translation. By reducing the number of unknowns during development, you can reduce the risk of having to revalidate your data or needing to repeat certain stages of the process. In the following blog, we outline how Rousselot Biomedical can help you reduce time to market by supporting you at every stage of development, from ideation through to final product.
1. Ideation and discovery research
Right from the start it is important to keep the end application in mind, as choosing pharmaceutical excipients that meet the requirements for every stage of the development of the product can save considerable time and effort. Our dedicated, expert team can help you to identify and optimize the ideal biomaterial for your research project. Due to our unique production process, all X-Pure® pharmaceutical grade gelatins and modified gelatins can be customized to meet the needs of your product; we can tailor the bloom, viscosity, molecular weight, degree of modification, or produce a whole new modification entirely. In addition, we offer technical and troubleshoot support throughout the development process.
2. Non-clinical safety assessment
Non-clinical safety assessment is a crucial checkpoint in the production of a biomedical product. Each ingredient in the formulation should be chosen carefully to ensure high quality and safety in order to minimize the risk of delays when moving from the lab into non-clinical safety assessment and beyond.
Choosing high-quality and high purity ingredients can help to reduce the risk of a product failing non-clinical safety assessments. Rousselot’s X-Pure range of pharmaceutical-grade, customizable, low endotoxin gelatins has been developed to ensure maximum quality and reliability.
How the use of Rousselot biomaterials can help save time: top qualities to look for when choosing a biomaterial
Biomaterial purity: One important factor to consider is the presence of impurities. Endotoxins can impact cell viability and proliferation and cause immune reactions which can influence preclinical research test results.
Biomaterial consistency: Batch to batch consistency allows for high reproducibility and controllability in terms of composition and biophysiochemical properties. Lower material variability means a higher signal-to-noise ratio for your trials.
Biomaterial customization: All X-Pure gelatins can be customized to meet the needs of your product; we can tailor the bloom, viscosity, molecular weight, degree of modification, or produce a whole new modification entirely.
Functional equivalence between research grade and GMP grade: Rousselot’s research and GMP grade gelatins are functionally equivalent, minimizing the need to revalidate data ahead of clinical trials and helping to accelerate research to clinic translation.
Contact us to customize a solution with X-Pure today.
3. Clinical trials – application and implementation
When a biomedical product reaches the clinical stage, it is crucial that all critical components are produced according to suitable Good Manufacturing Practices (GMP). This is to ensure the integrity of the manufacturing process, as well as compliance with safety regulations. Rousselot Biomedical is ISO 9001 certified and applies a voluntary compliance towards IPEC (International Pharmaceutical Excipients) GMP.
The launch of Rousselot’s GMP-grade X-Pure GelMA in April 2022 was a major step forward for companies that require GelMA for clinical use and in-body medical applications. Until then, research grade GelMA was the only available option. Production under IPEC GMP ensures that Rousselot X-Pure GelMAs are consistently produced and controlled according to strict quality standards, including consistent chemical modification and purification methods.
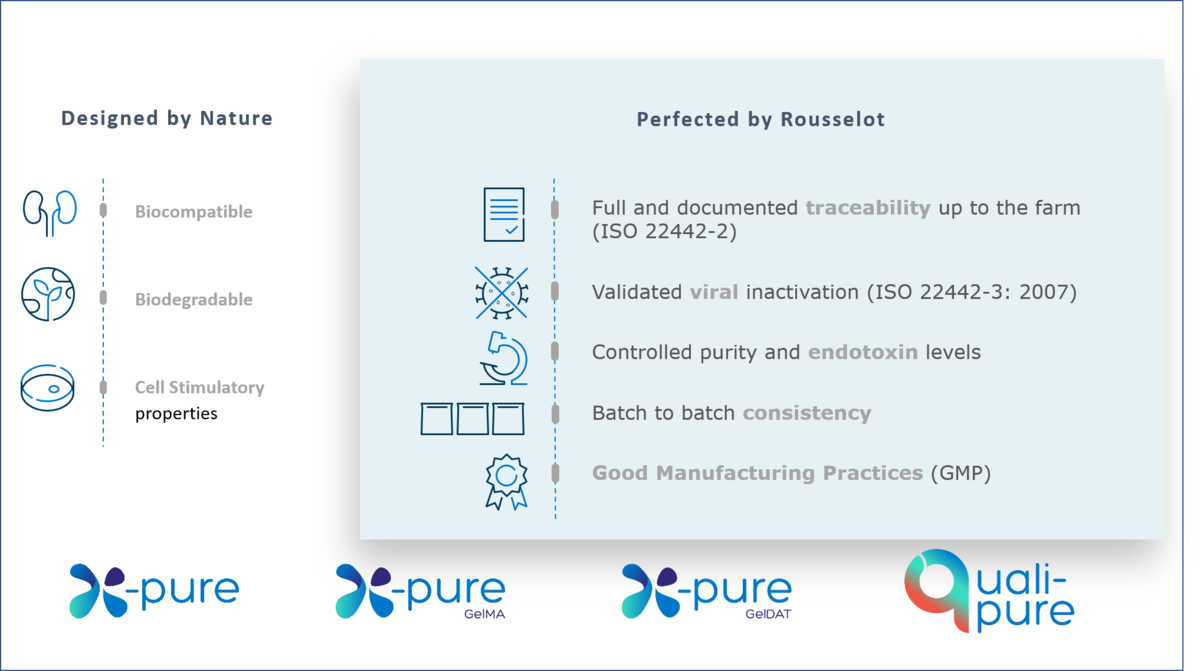
All X-Pure gelatins are highly biocompatible and support regulatory compliance with international pharmaceutical and medical device guidelines for ingredients or excipients of animal origin. In addition, they have ultra-low endotoxin levels (<10 (EU)/g), which are amongst the lowest available on the market – over 100 times lower than traditionally manufactured gelatins.

Rousselot’s innovative range of biomedical gelatins can unlock the pathway to clinical research
4. Product registration
To ensure smooth and efficient product registration, it is important to present proof that products and their components comply with market requirements. All X-Pure and Quali-Pure® gelatins and modified gelatins are carefully created for use in pharmaceutical or medical devices requiring compliance with global market standards and regulations. Our products come with supporting documentation for compliance of medical devices with MDR requirements ((EU) 2017/745), documented traceability up to the farm (ISO 22442-2), validated viral inactivation (ISO 22442-3) and IPEC GMP compliance. By providing this documentation, we can give you peace of mind and help you to avoid costly delays during the final stages of commercialization.
Choosing pharmaceutical excipients that comply with international safety regulations is crucial at the clinical and commercial stages of development
5. Final product: new medicine or medical device
Once your product is approved and the new medicine or medical device reaches market, there are several post approval obligations. Rousselot Biomedical stays on top of regulatory changes and can provide compliance documentation to ensure that our customers have everything they need to navigate the biomedical market.
Customers with approved medical devices on the EU market should be aware of the Medical Device Regulation (MDR) coming into force from May 2024. Regulation (EU) 2017/745 on Medical Devices (the MDR) has replaced the existing medical devices Directive (93/42/EEC) (MDD) and the active implantable medical devices Directive (90/385/EEC) (AIMDD). In addition, the ISO standard 22442 has been updated in 2020 to bring it further in line with global requirements for medical devices. Switching to a gelatin-based biomaterial that meet both the EU as ISO requirements can help to ensure the longevity of your product. All Rousselot Biomedical products have supporting documentation confirming compliance with the main pharmaceutical and medical device regulations worldwide.
Choose premium gelatins to give your biomedical application the best chance of success
In your development roadmap, it is crucial to consider the main regulatory requirements for the end product right from the start of development. Introducing consistency and minimizing risk early on can reduce the chances of having to revalidate your data between different stages of development, helping to smooth the transition from lab to clinic. Choosing high quality biomaterials that meet these criteria and support compliance with international regulations can help to give your biomedical application the best chance of success.
Read our blog, "Endotoxins, an inside story" to discover how purified gelatins can help to minimize data variability and improve repoducibility
Read our new study that confirms that purity and type of coating biomaterials used in 2D cell culturing are crucial for efficient stem cell proliferation
