Endotoxins
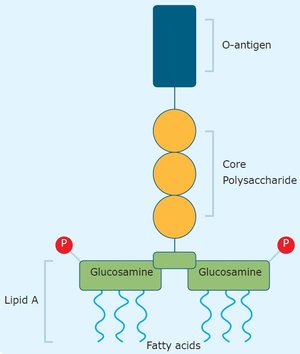
Gelatin and collagen are Generally Recognized As Safe (GRAS) by the US Food and Drug Administration (US FDA) and compliant with the US, European, and Japanese Pharmacopeia. But the use of traditionally manufactured gelatin and collagen - even of pharmaceutical and medical grades - in sensitive (bio)medical applications is challenged by the presence of endotoxins.
What are endotoxins?
Endotoxins or lipopolysaccharides are large, highly immunogenic molecules and the major component of the outer membrane of Gram-negative bacteria. For specific in-body biomedical applications, endotoxin limits have been defined to ensure patient safety. Complying with the limit values for endotoxins in these applications is a challenge: the endotoxin levels in traditionally manufactured gelatin and collagen are typically >100 times higher than those recommended by the United States’ Food and Drug Administration (USFDA), for example. Find out more about endotoxins in our latest blog, "Endotoxins: an inside story."
Did you know that 176 medical devices have been recalled by the FDA since 2005 due to high endotoxin levels…
Between 2005 and 1st of September 2021, the FDA recalled 176 medical devices owing to high levels of bacterial endotoxin. Patients exposed to bacterial endotoxin levels that are higher than those specified by the FDA for drugs and devices, could develop an acute inflammatory response.
Always select purified excipients to ensure your medical device or biomedical application for in-body use, has the lowest possible endotoxin content. X-Pure® gelatins are intensely purified, providing a safe biomaterial for applications development that supports rapid translation from lab to clinic.
The importance of low endotoxin levels for in-body applications and research

For in-body applications, even at low levels, endotoxins can pose a serious health risk. When exposed to the immune system, endotoxins initiate an immune response that can lead to tissue inflammation, increased sensitivity to other allergens, and the risk of fatal shock (Weil and Spink, 1957).
When it comes to pharma and medical research, working with low endotoxin or endotoxin-free materials is crucial. Low-endotoxin materials will create more effective manufacturing and research possibilities. For example, research has already shown improved cell viability and differentiation in endotoxin purified cell growth environment. Pharma and medical developers are continually developing new uses for gelatin and collagen-based applications, both human and veterinary.
X-Pure®: the first complete range of low-endotoxin gelatins and collagens
The X-Pure product range offers gelatins and collagens with extremely low endotoxin levels, consistent quality and traceability to the source for a wide range of applications.