Developments in regenerative medicine, facilitated by innovative biomaterials for cell culture, are enabling a new era of biomedical research and personalised medicines, reducing the need for animal testing and accelerating the delivery of life-changing treatments to patients.
Rousselot Biomedical produces GMP(1)-grade purified and modified gelatins for medical applications, where compliance with the relevant FDA and EU quality and safety regulations is key. Applications include a wide range of regenerative medicine applications, like 3D bioprinting, bone regeneration or implantable scaffolds. In this blog, we explain how to select the optimal gelatin-based biomaterial to ensure successful cell culturing.
Creating an optimal environment for cell growth
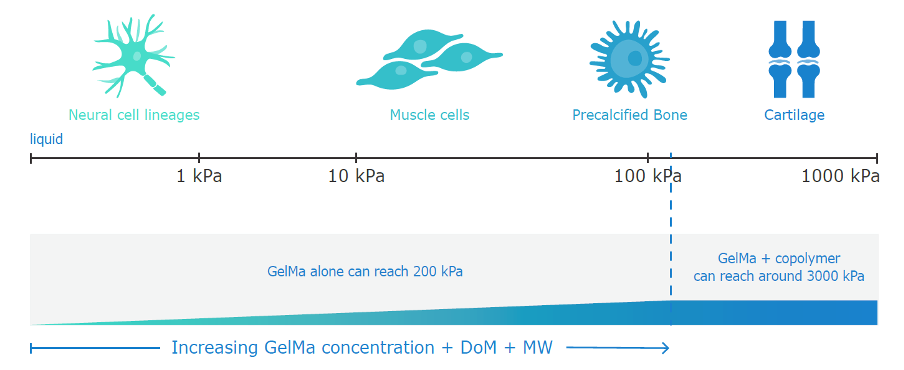
Cell-based or cell-laden therapies to repair, replace or regenerate injured and diseased tissues and organs require highly specialised cell culture conditions. Depending on the cells’ origins, it has different preferences for physicochemical properties – for example, liver cells prefer a softer environment to bone cells. The degree to which the cell culture conditions mimic the in vivo environment plays a critical role in enhancing cell survival, adhesion and functional performance.
One of the most versatile biomaterials used for 3D cell culture is gelatin-methacryloyl (GelMA). Rousselot’s X-Pure® GelMA offers tuneable mechanical properties that can be tailored by varying the degree of modification and the molecular weight. GelMA scaffolds can be further enriched with chemical properties and biological cues that are native to the tissue of interest.
The expert team at Rousselot Biomedical helps to formulate customised GelMA that mimics the strength, elasticity and porosity of the targeted natural cellular environment, whether it be skin, bones, or blood vessels. For example, Rousselot participates in the ENLIGHT project – a European collaboration that aims to develop a realistic 3D model of the pancreas to test new diabetes medications.
What characteristics should you look for when selecting a biomaterial for cell culture?
1. Purity
The first and key characteristic for any biomaterial used for tissue engineering is that it is safe to use in humans. Non-purified gelatin and GelMA hydrogels contain endotoxins which can initiate a strong immune response that leads to tissue inflammation, increased sensitivity to other allergens and the risk of fatal shock. Endotoxins also have implications at the research stage, where they can impact cell growth and differentiation. Choosing ultra-pure biomaterials such as X-Pure helps to overcome these problems. Rousselot’s process securely removes impurities, resulting in consistent and ultra-low impurity levels, making X-pure GelMA gelatins suitable for even the most sensitive applications.
2. Biocompatibility and biodegradability
Cells in our body are anchored to an extracellular matrix. Biomaterials derived from the extracellular matrix – like gelatin – are naturally biocompatible and biodegradable, making them ideal biomaterials for in body applications. Research has shown that gelatin can enhance the attachment and proliferation of cells promoting tissue repair.
3. Consistency and Scalability
Selecting a biomaterial with guaranteed batch-to-batch consistency at any scale, such X-Pure, can help avoid variability in your data. Using a biomaterial that has functional equivalence between research and GMP grade reduces the need to revalidate R&D results ahead of nonclinical or clinical trials, which is why in April 2022, Rousselot Biomedical announced the launch of the first commercially available GMP GelMA, a major step forward for companies that require GelMA for clinical use and in body medical applications.
4. Compliance
A crucial consideration for any biomaterial being used in medical applications is that it must be compliant with the applicable regulations and standards. All X-Pure gelatins are compliant to the requirements of the major pharmacopoeia and support regulatory compliance with the EU Medical Device Regulation (MDR) 2017/745 and ISO 22442. The materials come with full traceability, readily available documentation and validated viral inactivation, helping to save time when reaching the clinic.
Following these guidelines will help select the optimal gelatin-based biomaterial to ensure successful cell culturing, support regulatory requirements and ultimately accelerate the delivery of medicines to patients.
References
(1) IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients