Parenteral Formulation, vaccines
Today’s parenteral formulation industry is growing rapidly. In the vaccine space, government organizations worldwide are launching increasing numbers of programs to combat the rising prevalence of infectious diseases and the demand for excipients as safe carriers in vaccines and other injectables is higher than ever before [1].
Safe, biocompatible stabilizing agents in vaccines
Gelatin is widely used as a stabilizing agent in vaccines and parenteral formulations generally due to its high biocompatibility. Ultra-pure gelatin helps provide drug protection. High endotoxin levels – even in medical grade gelatins can risk provoking an unwanted immune reaction.
Rousselot HGP gelatins are the safe choice for vaccine formulation
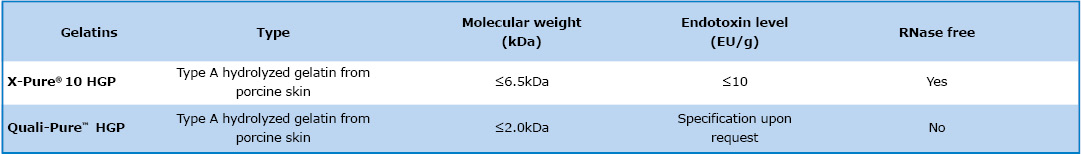
Our HGP hydrolyzed gelatins such as X-Pure 10HGP 6500 and Quali-Pure HGP 2000 are the safe choice for parenteral formulations. They help to stabilize vaccines and other formulations while minimizing the risk of endotoxin induced immune responses in drug delivery applications.
X-Pure and Quali-Pure come with readily available documentation and prolonged document retention, validated viral inactivation and Good Manufacturing Practices (GMP).
References:
[1] Kaddar M, Schmitt S, Makinen M, Milstien J. Global support for new vaccine implementation in middle-income countries. Vaccine. 2013 Apr 18;31 Suppl 2:B81-96.